Contents
- 1 Breakthrough in Diagnostic Technology: A Smartphone-Based Biosensor for Early Disease Detection
- 1.1 Why This Matters
- 1.2 How It Works
- 1.3 The Coffee-Ring Effect
- 1.4 Two-Step Process
- 1.5 Artificial Intelligence and Disease Patterns
- 1.6 Exceptional Sensitivity in Laboratory Testing
- 1.7 Accessible Technology Design
- 1.8 Future Applications and Challenges
- 1.9 Looking Ahead
- 1.10 Share this:
- 1.11 Related posts:
Breakthrough in Diagnostic Technology: A Smartphone-Based Biosensor for Early Disease Detection
A groundbreaking development from the University of California, Berkeley, has introduced a new type of biosensor that leverages smartphone cameras to detect disease proteins in just minutes. This innovative technology uses the coffee-ring effect and gold nanoparticles to identify even the smallest traces of disease markers in saliva or buffer samples.
Why This Matters
The prototype test demonstrates lab-based detection limits up to 30 times better than current hospital ELISA tests and over 100 times more sensitive than common lateral flow tests. This level of sensitivity could enable earlier intervention for conditions such as sepsis, COVID-19, and cancers, potentially saving lives by detecting these diseases at their earliest stages.
How It Works
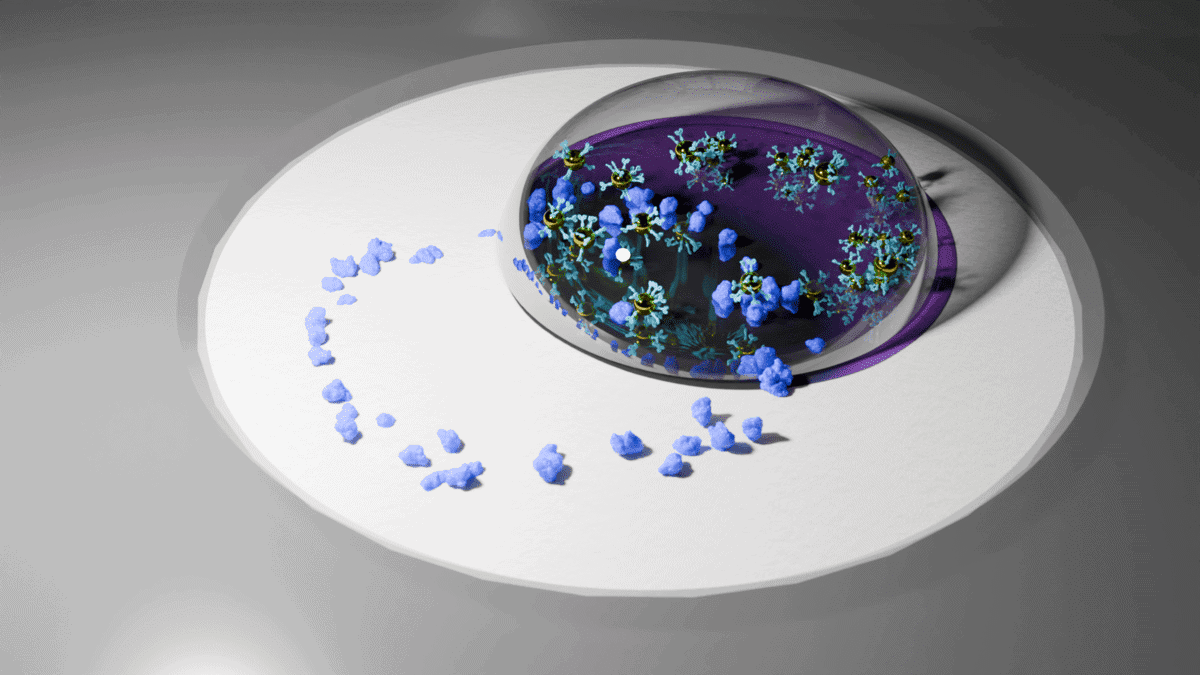
The process begins with a small liquid sample drying on a special membrane, which concentrates proteins into a visible ring pattern. A second droplet containing gold nanoshells binds to these proteins, forming distinct visual patterns. These patterns are then analyzed using artificial intelligence (AI) from a smartphone photo, allowing for quick and accurate diagnosis.
The Coffee-Ring Effect
The inspiration for this technology came from an everyday phenomenon—the coffee-ring effect. When spilled coffee dries, it naturally concentrates particles at the edges, creating those stubborn stains. Scientists realized they could use this same principle to magnify disease markers in samples. By mimicking this natural process, the Berkeley team created a method to enhance the sensitivity of a sensor.
Two-Step Process
The system works in two steps. First, a sample dries on a specially designed membrane, concentrating disease proteins into a ring pattern. Then, tiny gold particles with attached antibodies flow over these concentrated proteins, creating distinct visual patterns that reveal what diseases are present.
The entire process takes less than 12 minutes. AI then analyzes smartphone photos of these patterns, distinguishing between different diseases with remarkable accuracy.
Artificial Intelligence and Disease Patterns
Two AI systems work together to interpret test results. One acts like a screener, identifying whether disease markers are present. The other measures exact concentration levels to track disease progression over time.
During laboratory testing, the AI correctly identified positive cases across four different protein markers: SARS-CoV-2 nucleocapsid protein for COVID-19, procalcitonin for sepsis, and cancer markers PSA and CEA. The system maintained its accuracy even when tested with samples spiked into pooled human saliva.
Exceptional Sensitivity in Laboratory Testing
Laboratory testing revealed extraordinary sensitivity levels. For prostate-specific antigen (PSA), the test achieved a detection limit of 3 picograms per milliliter, about 30 times better than current ELISA tests used in hospitals. For COVID-19 protein detection, sensitivity surpassed lateral flow rapid tests by over 100 times.
Sepsis detection proved equally impressive. The test identified procalcitonin biomarkers at concentrations present just hours after infection begins, when current rapid methods typically fail. Since sepsis can progress rapidly to organ failure, this enhanced sensitivity could enable much earlier intervention.
Accessible Technology Design
Unlike existing diagnostic methods requiring expensive laboratory equipment and trained technicians, this system needs only a smartphone. Researchers designed the system for accessibility, though specific costs weren’t provided in the study.
The most sensitive component — the gold nanoparticle solution — remains stable for about six months when stored at 4°C. The team developed a complete testing kit using 3D-printed components and a battery-powered heating element.
Future Applications and Challenges
The technology goes beyond simple yes-or-no answers, providing precise measurements. The platform’s design allows new disease markers to be added by training the AI on additional proteins.
Laboratory testing with human saliva samples showed the system maintained its performance when detecting spiked proteins, demonstrating potential for real-world applications. However, the study was conducted entirely in laboratory settings using prepared samples rather than clinical testing with actual patients.
While the research shows promising laboratory results, the technology would need extensive clinical validation and regulatory approval before reaching patients. The authors acknowledge that different proteins require individual optimization, and the quality of commercial antibodies can affect performance.
Looking Ahead
The researchers have filed a U.S. patent based on this technology and are looking for possibilities to start a company concentrating on the development of this technology. They hope to begin a wide range of clinical tests with the at-home version of their sensor within a year, laying the groundwork for the next steps toward commercialization.
This innovation represents a significant step forward in the field of diagnostics, offering a low-cost, accessible solution for early disease detection that could transform healthcare, especially in low-resource or remote areas.